All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Venetoclax with HMA: A bridge to transplant in high-risk acute myeloid leukemia
High-risk acute myeloid leukemia (AML) is characterized by a high frequency of cytogenetic adverse-risk features which results in poor response to chemotherapy by patients.
Antiapoptotic B-cell lymphoma protein 2 (BCL-2) overexpression is associated with survival of AML cells and treatment resistance. In preclinical studies, venetoclax, which is an inhibitor of BCL-2, along with hypomethylating agents (HMAs) such as azacitidine, have been shown to induce cell death in AML-derived cell lines. In a previous study, which included elderly patients with intolerance to intensive chemotherapy, a synergistic treatment of venetoclax with HMA provided promising efficacy and a tolerable safety profile.1
Recently, at the 47th Annual Meeting of the EBMT, Federico V. et al. reported their single-center study to evaluate efficacy and safety of the combination of venetoclax and HMAs in patients with de novo or relapsed/refractory (R/R) AML.2
Patient demographics and baseline characteristics
In total, 24 patients were selected for the study with a median age of 69 (27−80 years). Table 1 includes details of patient demographics and baseline characteristics. Patients were divided into two groups. Patients in the de novo AML group (n = 14) had adverse cytogenic features and were not eligible for intensive chemotherapy treatment, but there was no incidence of relapse. The second group was the R/R AML group (n = 10), which included patients with incidence of relapse after receiving HMA (n = 3), AML induction therapy (n = 5), and allogeneic bone marrow transplant (n = 2).
Table 1. Patient demographics and baseline characteristics*
|
Allo-BMT, allogeneic bone marrow transplant; AML, acute myeloid leukemia; ECOG PS, Eastern Cooperative Oncology Group performance status; ELN, European LeukemiaNet; HMA, hypomethylating agent; R/R, relapsed/refractory. *Adapted from Federico V. et al.2 |
|||
|
Characteristic |
All patients |
De novo AML |
R/R AML |
|---|---|---|---|
|
Median age, years |
69 |
71 |
63 |
|
Sex |
|
|
|
|
ECOG PS, % |
|
|
|
|
ELN-2017 cytogenetic risk, % |
|
|
|
|
Peripheral blasts, % |
30 |
30 |
20 |
|
Bone marrow blasts, % |
55 |
40 |
60 |
|
Previous treatment, % |
|
|
|
Dosing schedule and treatment design
Each patient received a median of three cycles of treatment with a range of 1−19 cycles. Each cycle spanned for 28 days. All patients received venetoclax (100−400 mg) orally, while 20 mg/m2 decitabine was administrated intravenously to 15 patients, and 75mg/m2 of azacitidine was administered subcutaneously in nine patients. The treatment design and dosing schedule are presented in Figure 1.
Figure 1. Dosing schedule and treatment design for the study*
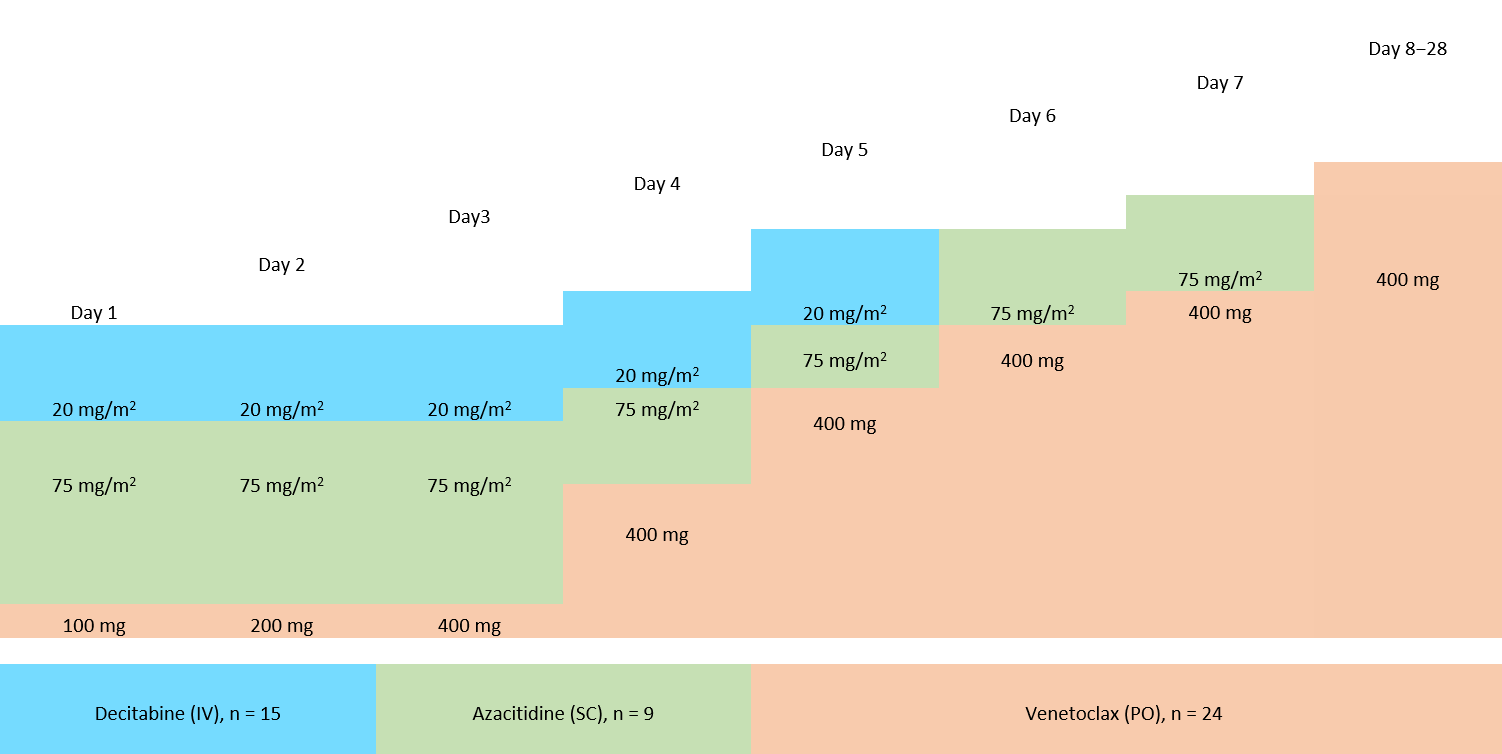
IV, intravenous; SC, subcutaneous; PO, oral administration.
*Adapted from Federico V. et al.2
Patient responses to the treatment
In the de novo AML cohort, the following aspects were observed: 78.5% of overall response rate, 50% of complete remission (CR), and 21.5% CR with incomplete marrow recovery. Median time to respond in this group was 2 months (Table 2).
In the R/R cohort, overall response rate was observed in 70% of patients, 30% had CR, and 20% of patients had CR with incomplete marrow recovery (Table 2).
Table 2. Response to the treatment*
|
AML, acute myeloid leukemia; CR, complete remission; iCR, CR with incomplete marrow recovery; PR, partial response. *adapted from Federico V. et al.2 |
|||
|
Response |
All patients |
De novo AML |
R/R AML |
|---|---|---|---|
|
Median number of cycles delivered |
3 |
3 |
3 |
|
Overall response rate, % |
79 |
78.5 |
70 |
|
No response, % |
20 |
21.5 |
30 |
|
Median time to response, months (range) |
1−5 |
1−5 |
1−5 |
Toxicity
Grade 3−4 toxicities included anemia (52%), thrombocytopenia (44%), leukopenia (62%), febrile neutropenia (61%), sepsis (32%), nausea (20%), and vomiting (12%). No deaths were observed during induction.
Transplant data
After a median of three cycles (range, 3−4) receiving HMA with venetoclax, nine patients (six with R/R AML, and three with de novo AML) underwent allogeneic stem cell transplantation (allo-SCT) with a mean cell dose of 6.3 × 106 kg. Further details on the characteristics of transplanted patients are summarized in Table 3.
After a median follow-up of 5 months (range, 2−22), 14 (58%) patients survived, 5 (21%) patients are still on therapy and in CR, and 10 (42%) patients had died of progressive disease.
Table 3. Patient characteristics for allo-SCT*
|
CR, complete remission; CTX +3, chemotherapy-treated for 3 days; CTX +5, chemotherapy-treated for 5 days; MMF, mycophenolate mofetil; CSA, cyclosporine A; iCR, CR with incomplete bone marrow recovery; R/R, relapse/refractory; TBF BU2, thiotepa-busulfan-fludarabine for 2 days; TBF BU3, thiotepa-busulfan-fludarabine for 3 days. *Adapted from Federico V. et al.2; †All values are in % except mentioned otherwise. |
|
|
Characteristic |
(%)† |
|---|---|
|
Median Age, years |
52 |
|
Cytogenetic profile |
|
|
Status at transplant |
|
|
Donor number |
|
|
Stem cell source |
|
|
Conditioning regimens |
|
|
GvHD prophylaxis |
|
|
GvHD |
|
Overall survival: transplanted vs nontransplanted patients
Significant overall survival (OS) improvements were observed for transplanted patients compared with nontransplanted patients; OS was 9.7 months in transplanted patients, and 3.0 months in nontransplanted patients (p = 0.001).
Conclusion
These short-term follow-up results indicate that venetoclax plus HMA can be a well-tolerated and effective combination for high-risk AML patients. Outcomes were particularly favorable in patients who underwent allo-SCT after HMA-venetoclax treatment (9.7 vs 3.0; p = 0.001). Thus, prior treatment of de novo or R/R AML patients who are referred for allo-SCT with HMA-venetoclax, could be a suitable option to attain better survival rates.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

