All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Secondary vs de novo acute myeloid leukemia
Do you know... Which of the following genes does not have high specificity (>95%) for secondary AML?
There are three main subtypes of acute myeloid leukemia (AML): de novo (primary) AML, which is the most common and arises as a new condition; around 20% of patients are diagnosed with secondary AML (sAML), which emerges alongside a history of hematologic disorders such as myelodysplastic syndromes (MDS) or myeloproliferative neoplasms (MPN); and therapy-related AML (t-AML), which is the least common form (6% of cases), and occurs following chemotherapy or radiotherapy treatment.1 During her presentation at the European School of Haematology (ESH) 8th Translational Research Conference: Myelodysplastic Syndromes, Felicitas Thol highlighted the major differences between these subtypes in terms of diagnosis, prognosis, and treatment guidance. We summarize the presentation below.2
Limitations of the current definition of sAML
Establishing a history of MDS or MPN can be difficult, as it requires a blood test or bone marrow biopsy prior to the development of AML.3 The new definition of AML with multilineage dysplasia by the World Health Organization (WHO) 2016 criteria is helpful as it also includes patients with MDS-associated cytogenetic changes, which are associated with adverse outcomes.1 However, some biologically important mutations involved in the clonal evolution from MDS to sAML are currently missing from this definition.1
Diagnosis
The importance of mutational analysis
Mutational analysis is helpful when diagnosing sAML, and can detect genetic changes that have driven the progression of MDS to AML. For instance, mutations in genes such as DNMT3A and TP53 are usually present in MDS, while FLT3 and other activating signal mutations are often acquired alongside AML progression.4
Further studies into drivers of the transformation of MDS to AML have found a number of associated mutational patterns. Genetic sequencing of 194 patients with sAML or t-AML revealed that the presence of mutations in SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, or STAG2 had high specificity (>95%) for sAML. Furthermore, patients with secondary-type mutations also had more recurrent driver mutations (p < 0.0001), and were older (p = 0.043), compared with patients diagnosed with de novo AML.5
Patients with MDS and a prior diagnosis of clonal hematopoiesis of indeterminate potential (CHIP) also progress to sAML in a mutation-dependent manner. Mutations in SRSF2, U2AF1, TP53, IDH1/2, and RUNX1 were found to be associated with a higher risk of progression, in a study of 95 patients.6
Prognosis
Patients with sAML and tAML show poorer survival compared to those with de novo AML. Furthermore, patients who have sAML arising from MPN have worse outcomes than patients with sAML arising from MDS.1 Intermediate-risk cytogenetics and being younger (≤60 years) also correlate with reduced survival.1 In addition, not only is sAML associated with adverse outcomes, but patients with de novo AML alongside secondary-type mutations have a poor event-free survival (EFS) and are less likely to achieve complete remission.5
Treatment guidance
With a number of promising novel therapies currently in clinical trials and coming to market, an understanding of AML subtype and the impact of this diagnosis on treatment decisions is increasingly important.
Gemtuzumab ozogamicin
Gemtuzumab ozogamicin (GO) is approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) following results from the ALFA-0701 trial. In a large meta-analysis,7 the benefit of gemtuzumab ozogamicin was only observed in patients with a favorable/intermediate cytogenetic profile, and patients with an unfavorable profile (e.g., AML with myelodysplastic-related changes/AML-MRC) were deemed unlikely to benefit from gemtuzumab ozogamicin.
CPX-351
In a phase II study, a comparison of CPX-351 with the standard chemotherapy regimen 7+3 demonstrated that patients with an adverse cytogenetic profile, or patients with sAML, gain the most benefit from treatment with CPX-351 (Figure 1).8
Figure 1. Complete response rates in patients with adverse cytogenetics and sAML treated with CPX-351 or 7+3 chemotherapy*
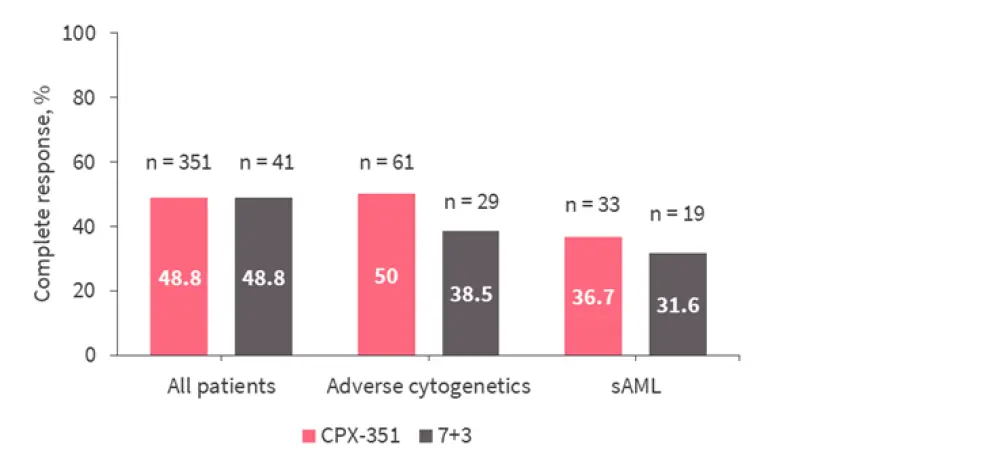
sAML, secondary AML.
*Adapted from Lancet et al.8
In a phase III trial conducted specifically in patients with AML-MRC or t-AML, CPX-351 led to superior overall survival (OS) in older patients (60–75 years old) compared with the 7+3 treatment (median, 9.56 vs 5.95 months; HR, 0.69; 95% CI, 0.52–0.90; p = 0.003), and prolonged EFS (median, 2.53 vs 1.31 months; HR, 0.74; 95% CI, 0.58–0.96; p = 0.021).9
Maintenance therapy
For older patients who are ineligible for stem cell transplantation, standard maintenance therapy is oral azacitidine (AZA; CC-486). The Quazar AML-001 trial showed that this agent provides both an overall and relapse-free survival benefit compared with placebo for patients in first complete response following chemotherapy. Subgroup analysis revealed that patients with history of MDS, chronic myelomonocytic leukemia (CMML) or poor-risk cytogenetics may also benefit from this maintenance therapy (Figure 2).10
Figure 2. 2-year OS with oral azacitidine or placebo in patients with history of MDS/CMML or poor-risk cytogenetics*
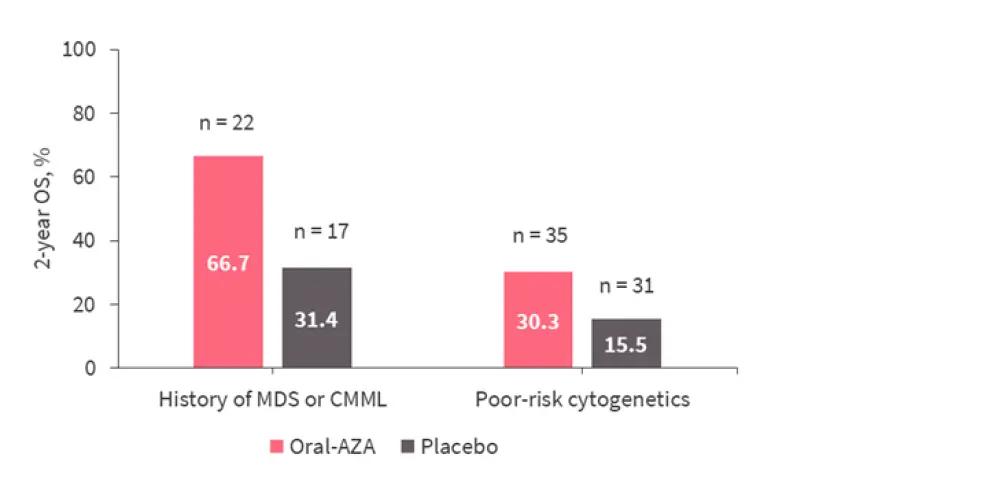
AZA, azacitidine; CMML, chronic myelomonocytic leukemia; MDS, myelodysplastic syndromes; OS, overall survival.
*Adapted from Wei et al.10
Induction therapy
Standard therapy for older patients is the azacitidine/venetoclax combination. Subgroup analysis of patients in the VIALE-A trial showed that the benefit of this regimen is not restricted to de novo AML, but can also benefit patients with sAML or poor-risk cytogenetics (Figure 3).11 However, this regimen may be less effective in patients with adverse cytogenetics—including complex karyotype, del(17p)—or TP53 mutations as they are more likely to be refractory to azacitidine/venetoclax or have clones arising after achieving remission (all factors associated with sAML).12
Figure 3. Survival in subgroups of patients with de novo AML or sAML, and intermediate- or poor-risk cytogenetics*
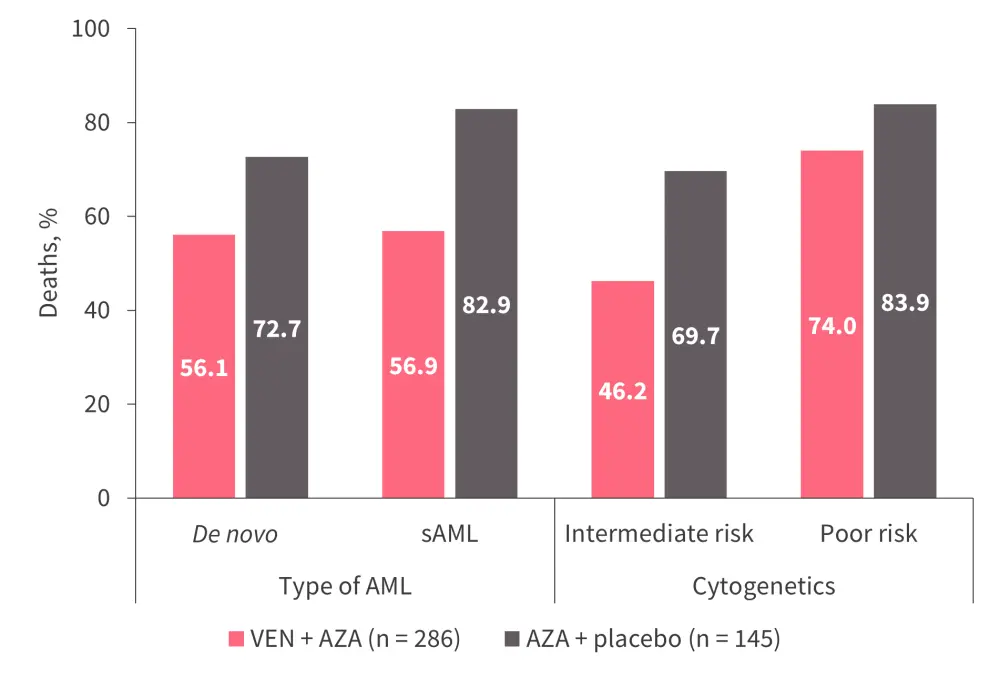
AML, acute myeloid leukemia; AZA, azacitidine; sAML, secondary acute myeloid leukemia; VEN, venetoclax.
*Adapted from DiNardo et al.11
Glasdegib, an oral smoothened receptor inhibitor, is another treatment option for patients ineligible for intensive chemotherapy, in addition to low-dose cytarabine (LDAC). When comparing long-term with LDAC alone, the benefit of this combination was more prominent in patients with sAML than in those with de novo AML (median OS, 9.1 vs 6.6 months).13
Measurable residual disease (MRD) in treatment evaluation
When treating sAML, usually the AML mutational clones are eradicated. However, in CHIP and MDS, mutations can remain, which impacts MRD monitoring. Most common are DNMT3A, TET2, and ASXL1 mutations, and so these should be excluded from MRD evaluation.14
Conclusion
Thol2 summarized that there are key clinical and biological differences between sAML and de novo AML, with a poorer prognosis seen in patients with sAML or sAML-type mutations. An understanding of the mutational landscape is helpful, as mutations in genes such as SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, and STAG2 are specific to sAML, and are therefore valuable in diagnosis. Fortunately, many of the newly approved therapies for AML are also efficacious in the sAML subtype; however, care needs to be taken with MRD evaluation regarding the presence of previous myeloid clones.
References

