All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Long-term analysis of phase II BRIGHT AML 1003 trial of glasdegib + LDAC in patients with AML who were ineligible for intensive chemotherapy
Acute myeloid leukemia (AML) is the most common type of leukemia in adults, and although most cases arise de novo, secondary AML (which arises due to antecedent myeloid disease, or as a late complication from chemo or radiation therapy) accounts for approximately 25% of all cases. Secondary AML usually occurs in the older population (70+ years) and is associated with lower response rates and decreased overall survival (OS) with standard chemotherapy compared with patients with de novo AML. Older patients are often ineligible for intensive chemotherapy because of comorbidities, performance status, or disease-related chemoresistance, and therefore, are treated with less aggressive therapies such as low-dose cytarabine (LDAC).1
The phase II BRIGHT AML 1003 trial (NCT01546038) aimed to evaluate the effect of using glasdegib, a potent, selective oral inhibitor of the Hedgehog signaling pathway, in combination with LDAC for the treatment of patients with AML who were ineligible for intensive chemotherapy. Indeed, this combination has already been approved in the newly diagnosed patient population in the US and Europe based on the results from this trial. The updated (>40-month) analysis from this trial was recently published by Michael Heuser and colleagues in the Annals of Hematology journal, and is summarized below.1
Study design and patient characteristics1
- Randomized, open-label, phase II trial in patients with previously untreated de novo or secondary AML who were not eligible to receive intensive chemotherapy
- 116 adult patients (≥ 55 years of age) were randomized 2:1 to receive glasdegib + LDAC (n = 78; n = 38 de novo AML, and n = 40 secondary AML) or LDAC alone (n = 38; n = 18 de novo AML, and n = 20 secondary AML)
- Glasdegib was administered at 100 mg, once daily, continuously for 28 days
- LDAC was administered at 20 mg/m2, twice daily, on Days 1–10 of a 28-day cycle
- Therapy was administered until disease progression, unacceptable toxicity, or patient refusal
- Primary endpoint: Overall survival (OS)
- Secondary endpoints: Response to treatment, tolerability, and toxicity
Baseline patient characteristics (Table 1) were generally similar across the treatment arms.
Table 1. Baseline patient characteristics*
|
AML, acute myeloid leukemia; ECOG PS, Eastern Cooperative Oncology Group performance status; ELN, European LeukemiaNet; Hb, hemoglobin; LDAC, low-dose cytarabine; MDS, myelodysplastic syndromes; sCr, serum creatinine; WBC, white blood cells. |
||
|
Characteristic |
Glasdegib + LDAC |
LDAC alone |
|---|---|---|
|
Sex, female, % |
24.4 |
39.5 |
|
Median age, years (range) |
77 (64–92) |
76 (58–83) |
|
Secondary AML, % |
||
|
Prior hematologic disease |
43.6 |
50.0 |
|
MDS |
37.2 |
39.5 |
|
Other |
6.4 |
10.5 |
|
Chemotherapy/radiotherapy |
7.7 |
2.6 |
|
Prior therapy with MDS drug, % |
||
|
Azacitidine |
12.8 |
13.2 |
|
Decitabine |
1.3 |
2.6 |
|
Median duration since diagnosis, months |
0.6 |
0.5 |
|
First-line AML nonintensive population criteria, % |
||
|
Age ≥75 years |
61.5 |
60.5 |
|
ECOG PS = 2 |
52.6 |
47.4 |
|
sCr > 1.3 mg/dL |
19.2 |
13.2 |
|
Severe cardiac disease |
66.7 |
52.6 |
|
ELN risk stratification for AML, % |
||
|
Favorable |
6.4 |
7.9 |
|
Intermediate I |
34.6 |
28.9 |
|
Intermediate II |
26.9 |
21.1 |
|
Adverse |
32.1 |
42.1 |
|
Mutations†, % |
|
|
|
FLT3 |
6.4 |
0 |
|
IDH1 or IDH2 |
24.3 |
15.8 |
|
NPM1 |
6.4 |
2.6 |
|
Median WBC count, 103/mm3 (range) |
2.7 (0.4–5,850.0) |
3.8 (1.2–1,370.0) |
|
Median Hb, g/dL (range) |
8.7 (6.9–13.8) |
9.0 (6.9–13.4) |
|
Median platelet count, 103/mm3 (range) |
42.0 (7.0–35,000.0) |
26.5 (3.0–23,000.0) |
|
Median % bone marrow blast (range) |
41.0 (16.0–99.0) |
46.0 (13.0–95.0) |
- The median duration of treatment in the glasdegib + LDAC arm was 83.0 days (range, 3–1,492) compared with 40.5 days (range, 6–239) in the LDAC alone arm.
- The median duration of treatment in the de novo AML subgroup with glasdegib + LDAC was 69.5 days (range, 5–1,206) and LDAC alone was 47.0 days (range, 10–239)
- The median duration of treatment in the secondary AML subgroup with glasdegib + LDAC was 101.0 days (range, 3–1,492) and LDAC alone was 39.0 days (range, 6–149).
Results1
Efficacy
- Median OS was superior in the glasdegib + LDAC arm compared with the LDAC alone arm, 8.3 months vs 4.3 months, respectively (HR; 0.495; 95% CI 0.325–0.752; p = 0.0004). When stratified by cytogenetic risk, this trend was consistent across most groups.
- The median OS in the de novo AML subgroup was 6.6 months vs 4.3 months, respectively (HR 0.720; 95% CI 0.395–1.312; p = 0.1398).
- This survival benefit was more pronounced amongst the secondary AML subgroup; median OS was 9.1 months vs 4.1 months, respectively (HR 0.287; 95% CI 0.151–0.548; p < 0.0001).
- More patients achieved a complete remission (CR) in the glasdegib + LDAC arm (19.2%) compared with the LDAC arm (2.6%). This trend was consistent for patients with de novo or secondary AML (Figure 1).
Figure 1. Percentage of patients achieving CR across different subgroups*
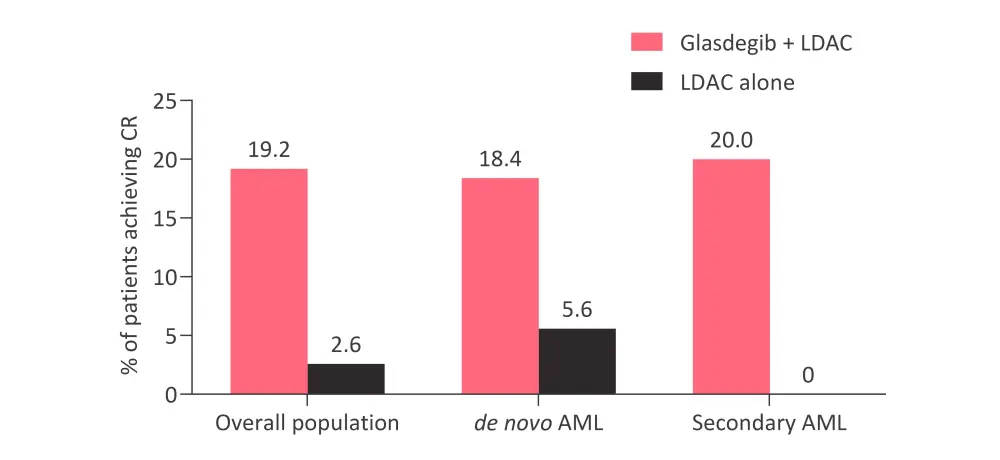
AML, acute myeloid leukemia; CR, complete response; LDAC, low-dose cytarabine.
*Data from Heuser et al.1
- The median duration of CR was longer in the glasdegib + LDAC arm (302 days) compared with the LDAC arm (91 days).
- and was also longer in the glasdegib + LDAC arm in patients with secondary AML (532 days) compared with de novo AML (175 days).
- Hematologic responses for the overall population can be seen in (Table 2).
- More patients in the glasdegib + LDAC cohort achieved durable (≥ 2 consecutive assessments) recovery of neutrophils, hemoglobin, and platelets, compared with the LDAC alone cohort.
- The median time to recovery of absolute neutrophil count was longer in the glasdegib + LDAC cohort compared with the LDAC alone cohort, while the median time to hemoglobin recovery was shorter.
- Patients in the glasdegib + LDAC arm also had fewer transfusions than those receiving LDAC alone and this was more significant when adjusted for the duration of treatment.
- More patients achieved transfusion independence in the glasdegib + LDAC cohort.
- Hematologic recovery and transfusion independence occurred at similar rates in the de novo and secondary AML subgroups.
Table 2. Hematological response in the overall population*
|
AML, acute myeloid leukemia; ANC, absolute neutrophil count; LDAC, low-dose cytarabine; PRBC, packed red blood cell. |
||||
|
Hematologic response |
Glasdegib + LDAC |
LDAC alone |
Glasdegib + LDAC |
LDAC alone |
|---|---|---|---|---|
|
ANC |
≥1000/μL |
≥500/μL |
||
|
All patients with recovery, % |
68.1 |
62.5 |
80.6 |
57.1 |
|
Patients with durable recovery†, % |
55.6 |
37.5 |
65.3 |
53.1 |
|
Median time to first recovery, days (range) |
27 (7–114) |
13 (8–70) |
16 (3–143) |
11 (8–119) |
|
Hemoglobin |
≥10 g/dL |
≥9 g/dL |
||
|
All patients with recovery, % |
59.7 |
56.3 |
88.9 |
68.8 |
|
Patients with durable recovery†, % |
31.9 |
21.9 |
61.1 |
40.6 |
|
Median time to first recovery, days (range) |
22 (6–129) |
33 (9–140) |
14 (4–172) |
22 (2–85) |
|
Platelets |
≥100,000/μL |
≥50,000/μL |
||
|
All patients with recovery, % |
50.0 |
21.9 |
68.1 |
40.6 |
|
Patients with durable recovery†, % |
41.7 |
12.5 |
52.8 |
25.5 |
|
Median time to first recovery, days (range) |
30 (6–171) |
26 (2–56) |
26 (4–141) |
24 (2–119) |
|
Transfusions, % |
(n = 75) |
(n = 36) |
— |
— |
|
No transfusions‡ |
29.3 |
5.6 |
— |
— |
|
PRBC transfusions |
33.3 |
8.3 |
— |
— |
|
Platelet transfusion |
42.7 |
11.1 |
— |
— |
|
Exposure-adjusted rate of any transfusion |
0.0696 |
0.1555 |
— |
— |
Safety
- The incidence of AEs was lower over the long term (>90 days) compared with the short term (<90 days) in both treatment arms.
- The most common treatment-emergent AEs were anemia and diarrhea in the glasdegib + LDAC cohort, and anemia, decreased appetite, and pneumonia in the LDAC alone cohort.
- Serious AEs (Grade ≥3) were reported in 80.0% and 77.8% of patients in the glasdegib + LDAC cohort and LDAC alone cohort, respectively (Table 3).
- The most common serious AEs in both groups were febrile neutropenia (28.0% and 16.7%, respectively) and pneumonia (21.3% and 19.4%, respectively).
Table 3. Grade ≥3 AEs occurring in ≥20% of patients*
|
AE, adverse event; LDAC, low-dose cytarabine. |
||||
|
AE |
Glasdegib + LDAC |
LDAC alone |
||
|---|---|---|---|---|
|
Grade 3–4 |
Grade 5 |
Grade 3–4 |
Grade 5 |
|
|
During the first 90 days, % |
||||
|
Any AEs |
70.7 |
16.0 |
55.6 |
36.1 |
|
Anemia |
41.3 |
0 |
36.1 |
0 |
|
Febrile neutropenia |
30.7 |
0 |
22.2 |
0 |
|
Thrombocytopenia |
30.7 |
0 |
22.2 |
0 |
|
Nausea |
1.3 |
0 |
2.8 |
0 |
|
Fatigue |
9.3 |
0 |
5.6 |
0 |
|
Peripheral edema |
0 |
0 |
2.8 |
0 |
|
Constipation |
1.3 |
0 |
0 |
0 |
|
Decreased appetite |
0 |
0 |
2.8 |
0 |
|
Dysgeusia |
0 |
0 |
0 |
0 |
|
Pyrexia |
1.3 |
0 |
2.8 |
0 |
|
Vomiting |
2.7 |
0 |
2.8 |
0 |
|
Pneumonia |
10.7 |
4 |
19.4 |
2.8 |
|
Diarrhea |
1.3 |
0 |
0 |
0 |
|
Dyspnea |
5.3 |
0 |
5.6 |
0 |
|
After 90 days, % |
||||
|
Any AEs |
51.2 |
23.3 |
42.9 |
21.4 |
|
Diarrhea |
7.0 |
0 |
7.1 |
0 |
|
Anemia |
23.3 |
0 |
21.4 |
0 |
|
Decreased appetite |
7.0 |
0 |
14.3 |
0 |
|
Muscle spasms |
9.3 |
0 |
0 |
0 |
|
Pyrexia |
2.3 |
0 |
7.1 |
0 |
|
Thrombocytopenia |
20.9 |
0 |
14.3 |
0 |
|
Nausea |
2.3 |
0 |
0 |
0 |
|
Neutropenia |
16.3 |
0 |
7.1 |
0 |
|
Pneumonia |
7.0 |
7.0 |
7.1 |
14.3 |
- Treatment discontinuation due to AEs occurred in 37.3% of patients that received glasdegib + LDAC and 47.2% in those who received LDAC alone.
- No patients discontinued treatment because of AEs associated with inhibition of Hedgehog signaling in normal tissues (e.g., muscle spasms, ageusia/dysgeusia, alopecia, weight loss, and asthenia).
Conclusions
The long-term analysis continued to demonstrate superior OS with glasdegib + LDAC compared with LDAC alone in patients with AML who were ineligible for intensive chemotherapy. This clinical benefit was more pronounced, statistically significant, and clinically meaningful in patients with secondary AML. The combination of glasdegib + LDAC was also particularly effective in severely neutropenic patients (those with an absolute neutrophil count ≥500/μL). Furthermore, treatment with glasdegib + LDAC was associated with an acceptable safety profile.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

