All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Expert safety recommendations and management for gemtuzumab ozogamicin treatment in AML
Gemtuzumab ozogamicin (GO) is a CD33-targeting monoclonal antibody that is conjugated to the cytotoxin, calicheamicin. Upon interaction with the CD33 marker on the surface of leukemic cells, it induces cytotoxicity and leads to tumor cell death.1 GO is currently approved by the U.S. Food and Drug Administration (FDA) for the treatment of adult and pediatric newly diagnosed CD33+ acute myeloid leukemia (AML) in combination with standard chemotherapy.1 Additional approvals include its use as monotherapy in the relapsed/refractory (R/R) CD33+ AML setting in both adult and pediatric patients (≥ 2 years old). In Europe, GO is licenced in combination with cytarabine and daunorubicin for the treatment of de novo CD33+ AML in previously untreated patients of ≥ 15 years old.1
Despite its good efficacy and established clinical use, GO is associated with multiple serious toxicities that require early identification and aggressive management in order to minimize poor patient outcomes. Such adverse events (AEs) include hepatotoxicity, myelosuppression, thrombocytopenia, infusion-related reactions (IRRs), and tumor lysis syndrome (TLS).1 GO-mediated hepatoxicity is usually accompanied by increased liver enzymes and hyperbilirubinemia but is mostly reversible. A more serious hepatic complication is the development of hepatic veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS), which commonly develops in patients after hematopoietic stem cell transplantation (HSCT).1
Jorge Cortes et al. published in the Journal of Hematology & Oncology1 a report of expert recommendations for the management and prevention of these serious GO-associated toxicities with a main focus on VOD/SOS. We hereby summarize that report and provide our readers with the latest expert advice on how to minimize and manage GO-related AEs.
Veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS)1
VOD/SOS is a very serious AE that often develops in patients who undergo HSCT, due to associated damage to hepatic sinusoids from the metabolism of conditioning regimens. This usually develops within one month following HSCT, and myeloablative conditioning along with advanced disease and extreme age (too young or too old) increase the risk for VOD/SOS development. According to the criteria set out by the European Society for Blood and Marrow Transplantation (EBMT), VOD/SOS is diagnosed on the basis of weight gain, as well as the existence of hepatomegaly, ascites, and increased bilirubin levels (Table 1).
Table 1. EBMT VOD/SOS criteria in adults and children1
|
*Best confirmed by imaging. |
|
|
Adults |
Children |
|---|---|
|
Classical (first 21 days after HSCT) Bilirubin ≥ 2 mg/dL + 2 of the following: Late onset (> 21 days after HSCT) Classical VOD/SOS beyond Day 21 |
No limitation for VOD/SOS time of onset The presence of ≥ 2 of the following: |
Treatment with GO has been associated with the development of VOD/SOS in both HSCT-receiving and non-receiving patients. Although the pathophysiology of this remains unclear, it has been suggested that the induction of cytotoxicity in potential CD33+ sinusoid epithelial cells might be the underlying cause. When comparing outcomes from older clinical trials, the risk of GO-mediated VOD/SOS today is much lower, most probably due to the movement towards smaller, fractionated GO doses and the improved awareness for VOD/SOS management. Nevertheless, it still remains a very important, life-threatening safety consideration that needs to be identified early and managed properly.
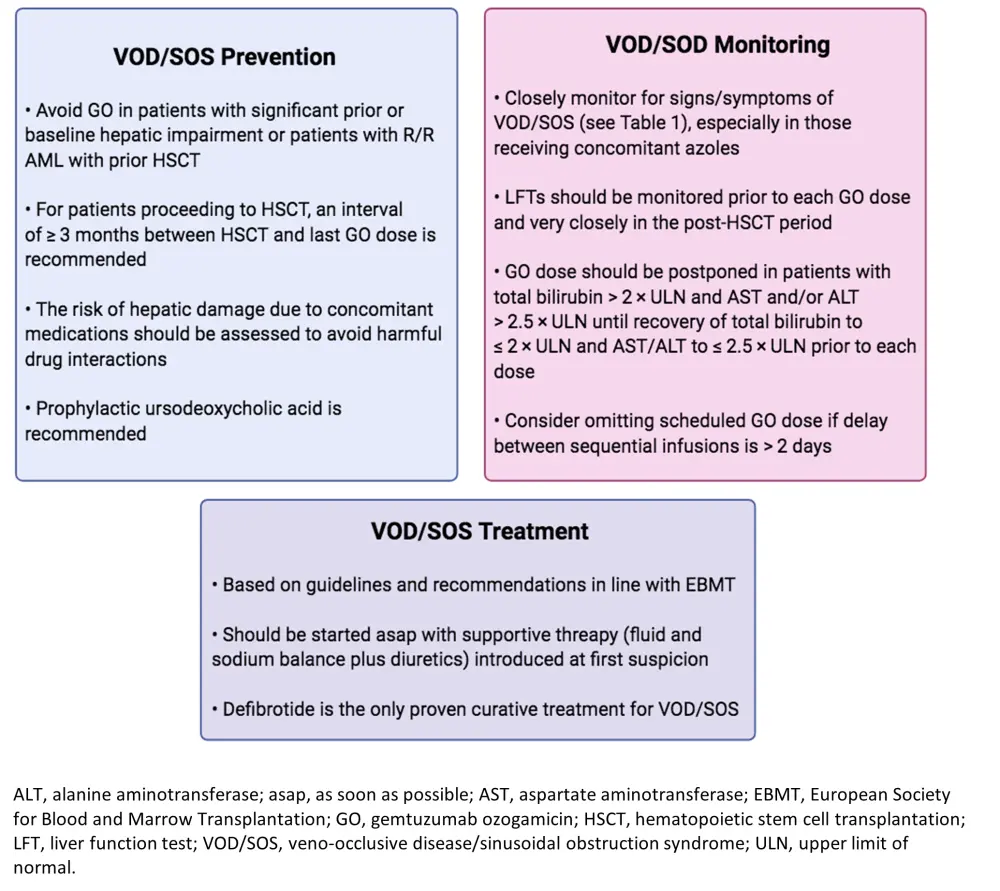
The current expert recommendations on VOD/SOS prevention and monitoring during GO treatment are shown below in Figure 1. In summary, patients with prior hepatic disease or damage (i.e., hepatitis, portal hypertension, cirrhosis, chronic liver disease), as well as patients with R/R AML who have undergone HSCT, are contraindicated for the use of GO unless the treatment will majorly benefit them. For patients that are scheduled to receive HSCT, a minimum wait period of 3 months should be implemented from the last GO dose. Although not get established, the authors also recommend the use of ursodeoxycholic acid as prophylaxis due to its potential of reducing toxic hydrophobic bile acids in the liver parenchyma. Patients with good-risk cytogenetics have a low risk of developing VOD/SOS, thus, GO is recommended in this patient subset. Nevertheless, the survival benefit of GO therapy seems to be decreased in those with intermediate-risk cytogenetics and completely abolished in those with high-risk cytogenetics.
With regards to VOD/SOS monitoring (Figure 1), the authors recommend close observation of patients receiving GO, especially those receiving concomitant azoles, for any associated signs and symptoms of VOD/SOS, which have been described in Table 1. It is critical to closely evaluate liver function prior each GO dose administration and especially in the post-HSCT period. Postponing or omitting GO treatment doses should be considered in cases of increased total bilirubin (> 2 × upper limit of normal [ULN]) and liver enzymes (> 2.5 × ULN) until their recovery. Moreover, clinicians could consider skipping GO doses if there has been a > 2-day delay between infusions.
VOD/SOS treatment recommendations have been published from the EBMT and state that treatment needs to be initiated upon the first indication of VOD/SOS with initial supportive fluid therapy and appropriate diuretics. Defibrotide is currently the only treatment for VOD/SOS and is recommended for both adult and pediatric patients. Recent evidence has suggested that defibrotide is also effective in treating HSCT-induced VOD/SOS.
Figure 1. Authors' recommendations for VOD/SOS prevention, treatment and monitoring1
Myelosuppression
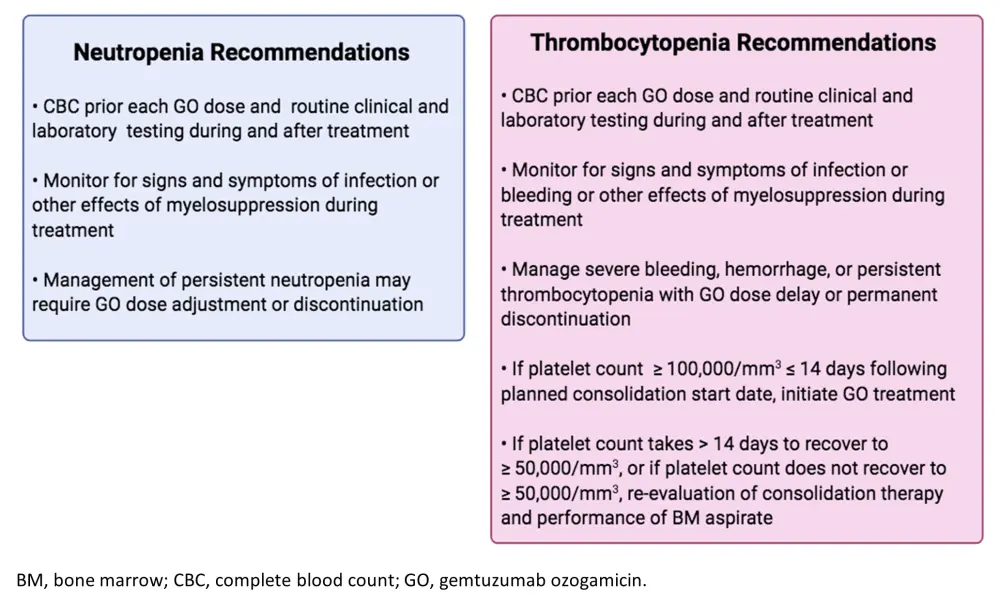
Myelosuppression is another commonly GO-associated AE. Prolonged neutropenia and thrombocytopenia have been observed following GO treatment. For managing these AEs, the authors recommend complete blood counts prior each GO dose and frequent monitoring for signs and symptoms of VOD/SOS, myelosuppression, or bleeding (Figure 2). In cases of severe, persistent neutropenia and thrombocytopenia (or bleeding), GO dose adjustment, delay, or discontinuation are recommended. In thrombocytopenia cases, if platelets fail to recover to ≥ 50,000/mm3 at all or in ≤ 14 days, then re-evaluation of treatment therapy and patient status via bone marrow aspirate are advised.
Figure 2. Recommendations for myelosuppression management and monitoring1
Infusion-related reactions (IRRs)
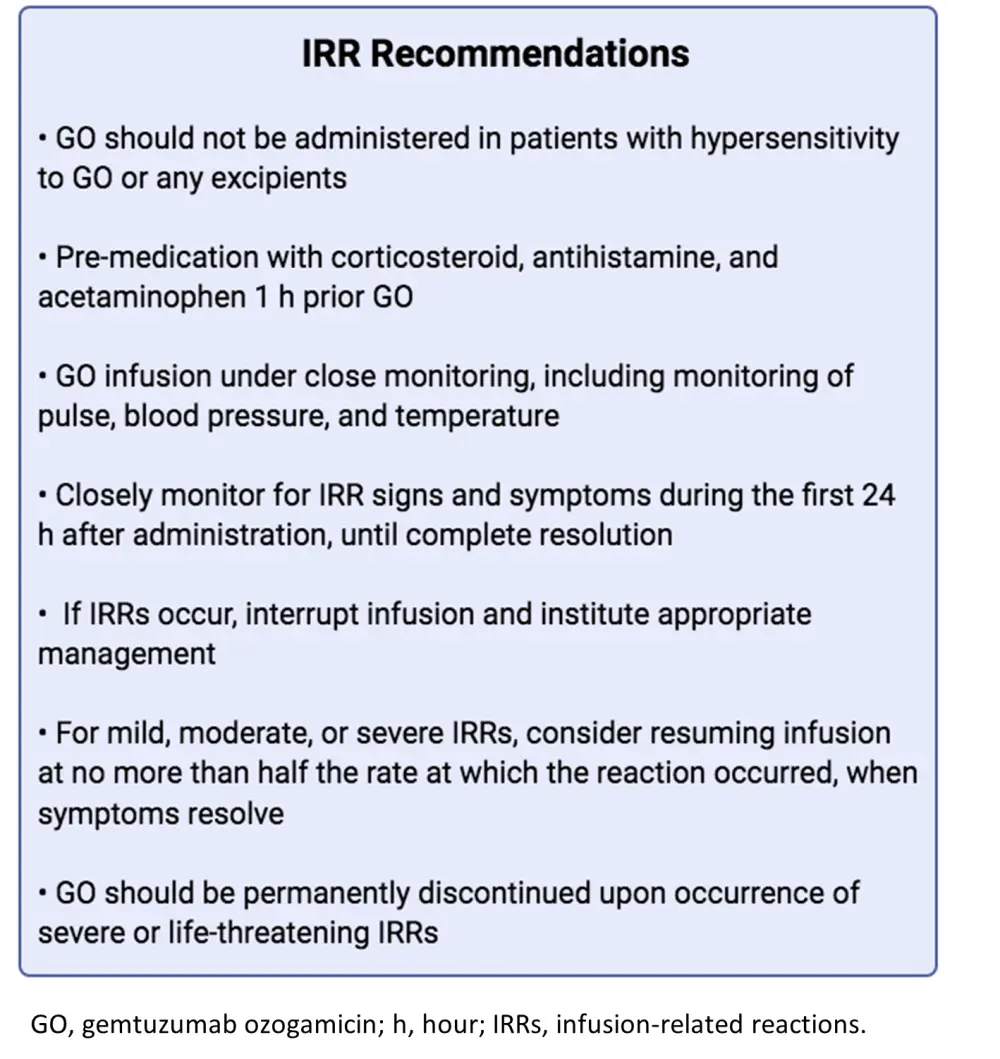
As a monoclonal antibody, GO can lead to IRRs and thus is contraindicated in patients with GO (or excipient) hypersensitivity. Reported GO-mediated IRRs are usually of mild nature and are easily manageable with supportive therapy. The first GO infusion is the most critical for the development of IRRs and should be closely monitored for signs, including pyrexia, tachycardia, hypotension, hypoxia, chills, and respiratory failure. The authors’ recommendation on the management and monitoring of GO-induced IRRs is shown below in Figure 3. These include pre-treatment with steroids, antihistamines, and acetaminophen 1 hour prior to GO administration and permanent GO discontinuation should severe or life-threatening IRRs occur.
Figure 3. Authors' recommendations for IRR management and monitoring1
Tumor lysis syndrome (TLS)
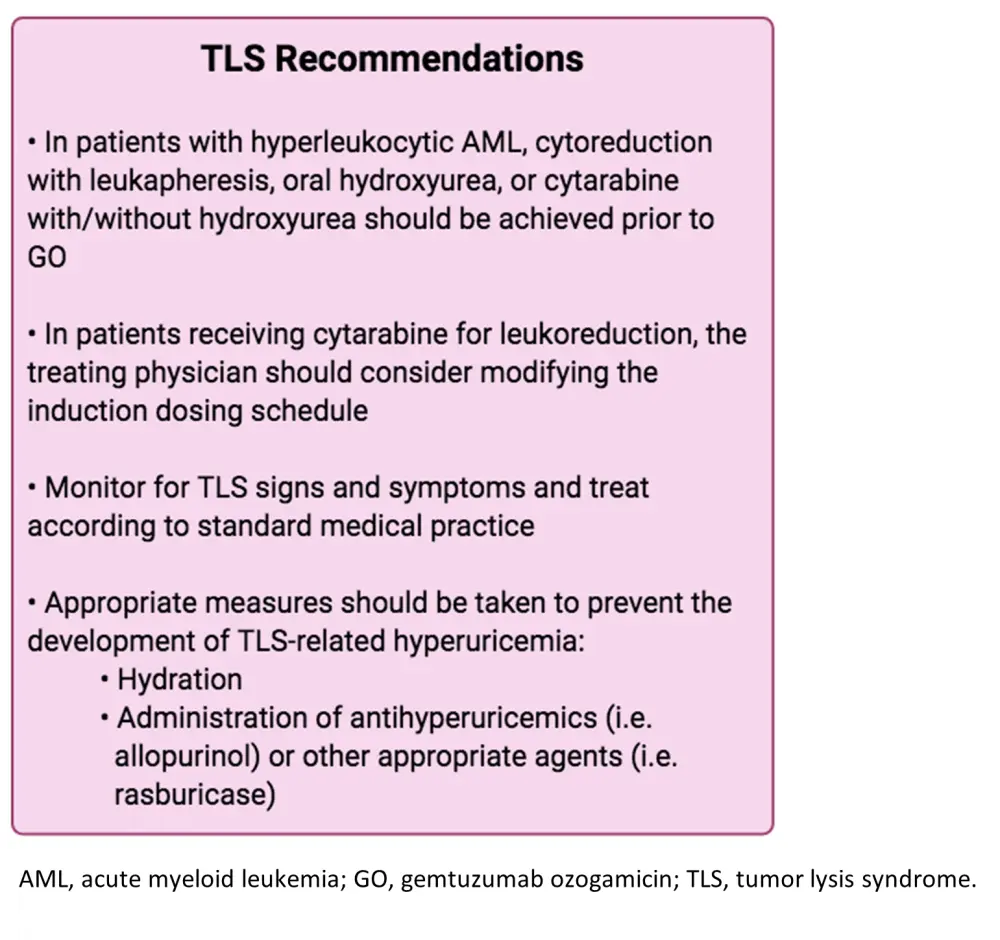
Last but not least, TLS has also been implicated following the use of GO for the treatment of leukemia. This is a serious AE that can lead to kidney damage, seizures, cardiovascular disturbances, and even death. According to the authors’ suggestions for TLS management (Figure 4), patients should be closely monitored for TLS signs and preventative measures like proper hydration and antihyperuricemics should be implemented prior to GO. Moreover, in patients with high white blood count or hyperleukocytic AML, appropriate cytoreductive procedures and agents should be successful prior to GO treatment. In cases where patients receive cytarabine for leukoreduction, GO induction dose modification should be considered.
Figure 4. Authors' recommendations for TLS management1
Conclusion
GO is an approved regimen for CD33+ naïve and R/R AML. Despite its promising efficacy in this setting, it has been associated with serious AEs, including VOD/SOS, myelosuppression, TLS, and IRRs. Current clinical data indicate that the risk of these serious toxicities has reduced over time. Nevertheless, they still pose a substantial risk to patient outcomes. The above summarized expert recommendations hope to aid with early identification and proper management of these toxicities, which are key to their resolution. In you want to find out more on the existing clinical data on GO, please read here.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

