All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Decitabine shows comparable OS to standard induction chemotherapy and better safety in patients with AML
Older patients diagnosed with acute myeloid leukemia (AML) who are fit for standard induction chemotherapy (IC) represent a high unmet clinical need due to secondary resistance to IC, and adverse genetics leading to poor long-term survival rates.1 Also, bridging to hematopoietic stem cell transplantation (HSCT) often fails. DNA-hypomethylating agents (HMAs) have been associated with reduced toxicity in patients unsuitable for IC, and patients with adverse genetics show a good response to HMAs.1
For these reasons, Lübbert et al.1 investigated the efficacy and safety of a 10-day decitabine regimen compared to standard IC (“3 + 7”), followed by allografting as a bridging therapy to transplant in older, fit patients with AML. Lübbert et al. presented their findings at the European Hematology Association (EHA) 2022 Congress, and we summarize the results below.
Study design
The international, open-label, randomized phase III trial (NCT02172872) had the following inclusion criteria:
- Age ≥60 years
- Eligible for standard IC
- Newly diagnosed de novo or secondary, untreated AML
- White blood cell count ≤30 × 109/L at randomization
The overall study design is shown in Figure 1.
Figure 1. Study design*
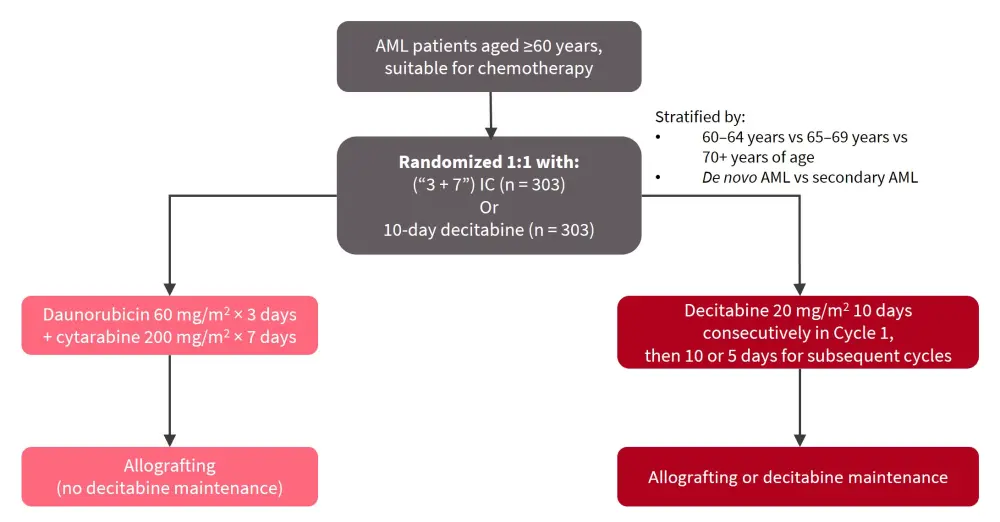
AML, acute myeloid leukemia; IC, induction chemotherapy.
*Adapted from Lübbert, et al.1
The primary endpoint for the study was overall survival (OS). The secondary endpoints were complete response rate/complete response with incomplete hematologic recovery (CR/CRi), progression-free survival, disease-free survival, transplant rate and outcome, safety, quality of life, and health economics.
Results
A total of 606 patients were included in the study, with 303 patients in each treatment arm (Table 1). Patient characteristics were comparable between the two arms.
Table 1. Patient characteristics*
|
ECOG, Eastern Cooperative Oncology Group; ELN, European Leukemia Network; MK, monosomal karyotype. |
||
|
Characteristic |
Decitabine |
“3 + 7” regimen |
|---|---|---|
|
Median age, years |
67 |
68 |
|
60–64, % |
25 |
25 |
|
65–69, % |
42 |
41 |
|
≥70, % |
33 |
34 |
|
ECOG performance status, % |
||
|
0 |
50 |
52 |
|
1 |
42 |
40 |
|
2 |
8 |
8 |
|
ELN 2017 risk group, % |
||
|
Favorable |
25 |
17 |
|
Intermediate |
45 |
48 |
|
Adverse |
30 |
35 |
|
Cytogenetics, % |
||
|
Normal karyotype |
53 |
45 |
|
Adverse karyotype, MK− |
32 |
40 |
|
MK+ |
15 |
15 |
|
Mutations, % |
||
|
NPM1 |
23 |
14 |
|
TP53 |
18 |
18 |
Patients who were treated with decitabine received a median of three cycles, while patients who were treated with IC received a median of two cycles. HSCT was performed in 122 patients (40%) in the decitabine arm, and 118 patients (39%) in the IC arm.
The median follow-up duration was 4 years. The OS was not significantly different between the two treatment arms (HR, 1.04; 95% confidence interval [CI], 0.86–1.26; 2-sided p = 0.68). The OS rates at 1, 2, 3, and 4 years for the two treatment arms are shown in Table 2. The 4-year incidence of relapse/progressive disease and treatment-related mortality in the decitabine arm was 57% and 21%, respectively, compared with 51% and 25% in the “3 + 7” IC arm, respectively.
Table 2. OS rates in the different arms after 1, 2, 3, and 4 years*
|
OS, overall survival. |
||||
|
Treatment arm |
OS, % |
|||
|---|---|---|---|---|
|
Year 1 |
Year 2 |
Year 3 |
Year 4 |
|
|
Decitabine |
58 |
37 |
30 |
26 |
|
Induction chemotherapy |
59 |
40 |
33 |
30 |
The CR/CRi rate for decitabine during on-protocol therapy was 48% compared to 61% for IC, while the overall CR/CRi rates were 60% and 67%, respectively.
The subgroup analysis of OS indicated that:
- Younger patients benefited more from the “3 + 7” IC regimen, while older patients benefited from the decitabine regimen.
- Patients classified as “favorable” according to the ELN-2017 risk group benefited more from the “3 + 7” IC regimen, while patients classified as “adverse” benefited from decitabine.
- Patients with mutated NPM1 benefited more from the “3 + 7” IC regimen, while patients with the wild type had no difference between either regimen.
- Patients who were monosomal karyotype-positive benefited more from decitabine, whereas patients who were monosomal karyotype-negative benefited from the “3 + 7” IC regimen.
Following allo-HSCT, the OS rates at 1, 2, 3, and 4 years, and the 4-year incidence of progression-free survival and treatment-related mortality were similar between the two arms.
Safety
The incidence of Grade 5 treatment-related adverse events recorded after HSCT was comparable between the two treatment arms, at 25% and 22% in the decitabine and IC arms, respectively. Time to Grade ≥3 infections and gastrointestinal adverse events appeared to be shorter with the “3 + 7” IC regimen compared with decitabine. The incidence of Grade 3–5 adverse events was higher with the “3 + 7” IC regimen, with the most common events being:
- blood and lymphatic disorders
- infections
- laboratory investigations
- gastrointestinal disorders
Health economics
The decitabine regimen was associated with statistically significant differences in terms of shorter hospitalization (p = 0.007), less need for intravenous antibiotics (p < 0.001), and transfusion (red blood cell units, p = 0.024; and platelet units, p < 0.001).
Conclusion
Both treatment arms recorded similar OS and HSCT rates. However, patients aged ≥70 years had a longer OS with decitabine, while younger patients (aged 60–64 years), or those with NPM1 mutations, had a longer OS with the “3 + 7” IC regimen. In terms of response rate, decitabine recorded a lower CR/CRi rate versus the “3 + 7” regimen; however, the safety profile and health economics profile were more favorable. The overall transplant rates (both on- and off-protocol) were >50% in both arms.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

