All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
A step forward to implement leukemic stem cell assessment in the diagnostic workup of AML
Leukemic stem cells (LSCs) have been strongly associated with residual disease and relapse in patients with acute myeloid leukemia (AML). A higher frequency of LSCs at diagnosis is correlated with the detection of measurable residual disease and poor prognosis; therefore, its assessment could lead to risk stratification at diagnosis and help in treatment decisions. However, before establishing a new diagnostic protocol in clinical practice, it must be practiced and validated in multiple centers.
To achieve LSC assessment in a globally standardized and validated way, Diana Hanekamp and colleagues designed a study to evaluate the technical and analytical feasibility of an eight-color LSC single tube assay technique in different laboratories from Europe and the United States. Their methodology and results have been recently published in the British Journal of Haematology.1
Methods
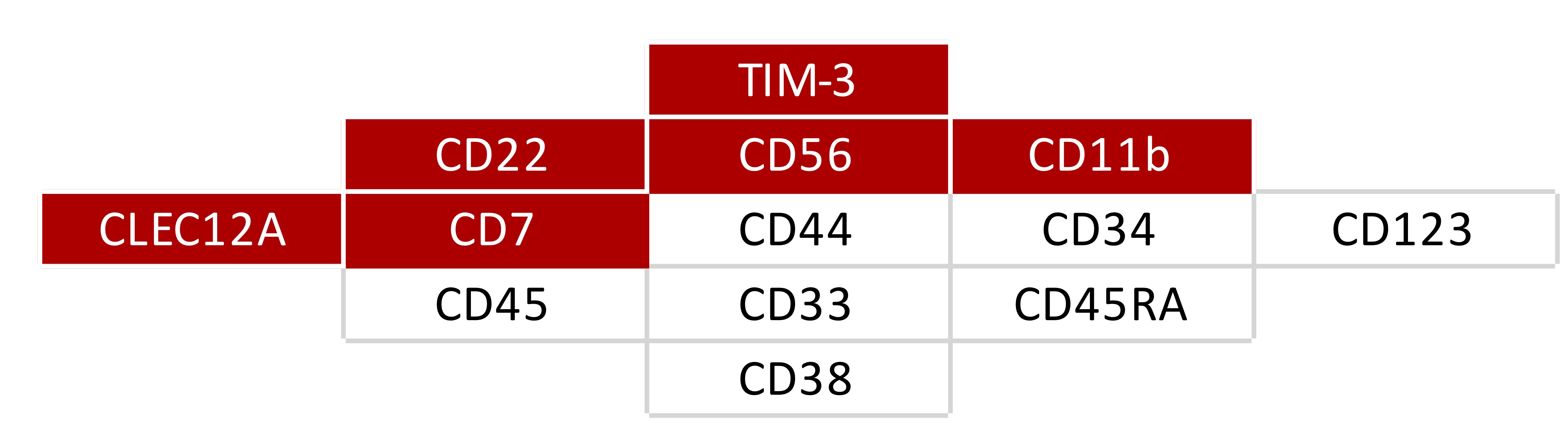
First, the authors established a panel to immunophenotypically identify LSCs. In addition to antigens commonly assessed in the clinic (i.e., CD34 and CD38), it was necessary to include 11 other markers to discriminate between LSCs and healthy hematopoietic stem cells. The final panel (Figure 1) was arranged in a single eight-color flow cytometry antibody panel and tested in a large cohort of patients with AML.
Figure 1. Selected LSC markers. In red, the markers combined in one fluorescence channel ("Combi" channel).1
The following steps were designed to evaluate the technical and analytical feasibility, as well as standardization, of the eight-color LSC single-tube assay:
- Setup of flow cytometers based on EuroFlow instructions
- Training at the central site and local sites
- Three cryopreserved diagnosis samples were used to evaluate inter-instrument variance, and four cryopreserved diagnosis samples for inter-laboratory processing
- Researchers from the central site first analyzed the results obtained by local sites and subsequently trained local researchers to analyze their results
- Variability was observed in data analysis in local sites, but not in data collection
- Standardization of the gating strategy
- Training with the diagnosis samples used above
- The critical steps identified were the gating of the white blood cell compartment, and discrimination of CD34+ blasts from CD34- blasts
- Gating validation
- The previous methodology was validated in 10 Flow Cytometry Standard files of representative diagnosis samples from patients with AML generated in the central site
Previously published studies identified a cutoff of 0.03% of LSCs to be clinically and prognostically significant.2 In line with these results, samples were classified as “low” or “high” according to the percentage of LSCs detected:
- LSChigh ≥ 0.03%
- LSClow < 0.03%
During the validation procedure, results from all trained researchers coincided for 13 of 14 samples (93%) in the discrimination between LSChigh and LSClow, including two samples that were around the cutoff value and could be easily misinterpreted.
The participating researchers chose CD45RA as the best LSC marker in 63% of cases, and although there was some variability in the exact percentages of LSCs, it did not impede categorizing samples as LSChigh, which is the variable with demonstrated clinical value. There was a positive correlation between local sites and the central site in the LSC burden assessment (mean r = 0.999; range, 0.998–1.000; p < 0.001).
Conclusion
With the improved techniques to detect measurable residual diseases, it is possible to identify low levels of persisting disease in patients with AML in remission. These remaining cells are potentially LSCs and represent underlying leukemia propagation, therapy resistance, and, consequently, AML relapse.
The LSC burden assessment could have a significant clinical impact, allowing treatment teams to stratify patients’ risk of relapse at diagnosis, leading to earlier identification of those with a poorer prognosis (LSChigh).
The one-tube LSC assay proposed and validated by Hanekamp et al. could be implemented easily in centers with relevant experience in flow cytometry after appropriate training. However, the authors highlight the importance of further evaluation in prospective multicenter studies to test the procedure in the context of the heterogeneity usually seen in AML.
This study reiterates the importance of the harmonization between flow cytometers, the standardization of the antibody panel, and the definition of a common gating strategy to be able to compare results between centers.
To further understand the critical role of LSCs in AML progression and also as a therapeutic target, read the related articles on the AML Hub.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

